- Calcium & bone metabolism
- Persistence with Denosumab in Male Osteoporosis Patients: A Real-World, Non-Interventional Multicenter Study
-
Chaiho Jeong, Jeongmin Lee, Jinyoung Kim, Jeonghoon Ha, Kwanhoon Jo, Yejee Lim, Mee Kyoung Kim, Hyuk-Sang Kwon, Tae-Seo Sohn, Ki-Ho Song, Moo Il Kang, Ki-Hyun Baek
-
Endocrinol Metab. 2023;38(2):260-268. Published online April 27, 2023
-
DOI: https://doi.org/10.3803/EnM.2023.1663
-
-
1,763
View
-
109
Download
-
1
Crossref
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material PubReader PubReader  ePub ePub
- Background
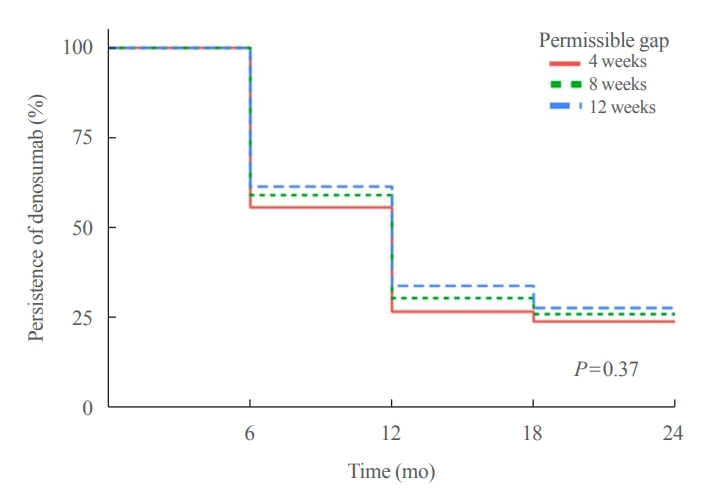
Persistence with denosumab in male patients has not been adequately investigated, although poor denosumab persistence is associated with a significant risk of rebound vertebral fractures.
Methods
We retrospectively evaluated 294 Korean male osteoporosis patients treated with denosumab at three medical centers and examined their persistence with four doses of denosumab injection over 24 months of treatment. Persistence was defined as the extent to which a patient adhered to denosumab treatment in terms of the prescribed interval and dose, with a permissible gap of 8 weeks. For patients who missed their scheduled treatment appointment(s) during the follow-up period (i.e., no-shows), Cox proportional regression analysis was conducted to explore the factors associated with poor adherence. Several factors were considered, such as age, prior anti-osteoporotic drug use, the treatment provider’s medical specialty, the proximity to the medical center, and financial burdens of treatment.
Results
Out of 294 male patients, 77 (26.2%) completed all four sequential rounds of the denosumab treatment. Out of 217 patients who did not complete the denosumab treatment, 138 (63.6%) missed the scheduled treatment(s). Missing treatment was significantly associated with age (odds ratio [OR], 1.03), prior bisphosphonate use (OR, 0.76), and prescription by non-endocrinologists (OR, 2.24). Denosumab was stopped in 44 (20.3%) patients due to medical errors, in 24 (11.1%) patients due to a T-score improvement over –2.5, and in five (2.3%) patients due to expected dental procedures.
Conclusion
Our study showed that only one-fourth of Korean male osteoporosis patients were fully adherent to 24 months of denosumab treatment.
-
Citations
Citations to this article as recorded by  - Denosumab
Reactions Weekly.2023; 1963(1): 206. CrossRef
- Diabetes, Obesity and Metabolism
- Effect of the Concomitant Use of Subcutaneous Basal Insulin and Intravenous Insulin Infusion in the Treatment of Severe Hyperglycemic Patients
-
Yejee Lim, Jung Hun Ohn, Joo Jeong, Jiwon Ryu, Sun-wook Kim, Jae Ho Cho, Hee-Sun Park, Hye Won Kim, Jongchan Lee, Eun Sun Kim, Nak-Hyun Kim, You Hwan Jo, Hak Chul Jang
-
Endocrinol Metab. 2022;37(3):444-454. Published online June 3, 2022
-
DOI: https://doi.org/10.3803/EnM.2021.1341
-
-
59,042
View
-
240
Download
-
3
Web of Science
-
3
Crossref
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material PubReader PubReader  ePub ePub
- Background
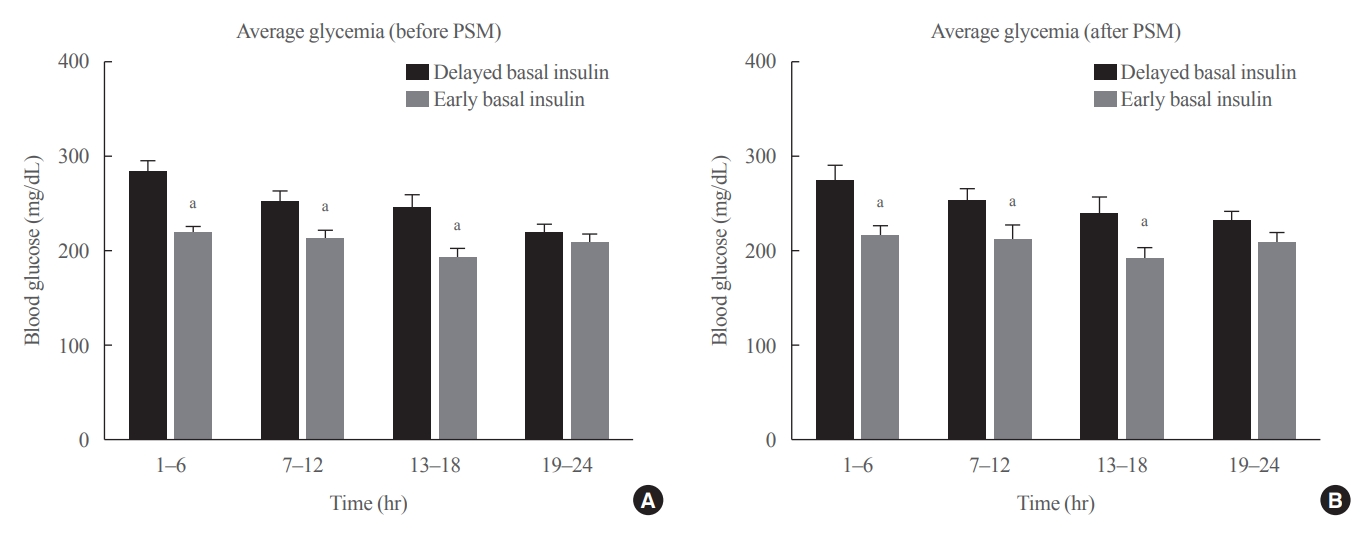
No consensus exists regarding the early use of subcutaneous (SC) basal insulin facilitating the transition from continuous intravenous insulin infusion (CIII) to multiple SC insulin injections in patients with severe hyperglycemia other than diabetic ketoacidosis. This study evaluated the effect of early co-administration of SC basal insulin with CIII on glucose control in patients with severe hyperglycemia.
Methods
Patients who received CIII for the management of severe hyperglycemia were divided into two groups: the early basal insulin group (n=86) if they received the first SC basal insulin 0.25 U/kg body weight within 24 hours of CIII initiation and ≥4 hours before discontinuation, and the delayed basal insulin group (n=79) if they were not classified as the early basal insulin group. Rebound hyperglycemia was defined as blood glucose level of >250 mg/dL in 24 hours following CIII discontinuation. Propensity score matching (PSM) methods were additionally employed for adjusting the confounding factors (n=108).
Results
The rebound hyperglycemia incidence was significantly lower in the early basal insulin group than in the delayed basal insulin group (54.7% vs. 86.1%), despite using PSM methods (51.9%, 85.2%). The length of hospital stay was shorter in the early basal insulin group than in the delayed basal insulin group (8.5 days vs. 9.6 days, P=0.027). The hypoglycemia incidence did not differ between the groups.
Conclusion
Early co-administration of basal insulin with CIII prevents rebound hyperglycemia and shorten hospital stay without increasing the hypoglycemic events in patients with severe hyperglycemia.
-
Citations
Citations to this article as recorded by  - 16. Diabetes Care in the Hospital: Standards of Care in Diabetes—2024
Nuha A. ElSayed, Grazia Aleppo, Raveendhara R. Bannuru, Dennis Bruemmer, Billy S. Collins, Laya Ekhlaspour, Rodolfo J. Galindo, Marisa E. Hilliard, Eric L. Johnson, Kamlesh Khunti, Ildiko Lingvay, Glenn Matfin, Rozalina G. McCoy, Mary Lou Perry, Scott J.
Diabetes Care.2024; 47(Supplement): S295. CrossRef - 16. Diabetes Care in the Hospital: Standards of Care in Diabetes—2023
Nuha A. ElSayed, Grazia Aleppo, Vanita R. Aroda, Raveendhara R. Bannuru, Florence M. Brown, Dennis Bruemmer, Billy S. Collins, Marisa E. Hilliard, Diana Isaacs, Eric L. Johnson, Scott Kahan, Kamlesh Khunti, Jose Leon, Sarah K. Lyons, Mary Lou Perry, Priya
Diabetes Care.2023; 46(Supplement): S267. CrossRef - Effectiveness and safety of early insulin glargine administration in combination with continuous intravenous insulin infusion in the management of diabetic ketoacidosis: A randomized controlled trial
Kitti Thammakosol, Chutintorn Sriphrapradang
Diabetes, Obesity and Metabolism.2023; 25(3): 815. CrossRef
- Bone Metabolism
- Comparison of the Effects of Various Antidiabetic Medication on Bone Mineral Density in Patients with Type 2 Diabetes Mellitus
-
Jeonghoon Ha, Yejee Lim, Mee Kyoung Kim, Hyuk-Sang Kwon, Ki-Ho Song, Seung Hyun Ko, Moo Il Kang, Sung Dae Moon, Ki-Hyun Baek
-
Endocrinol Metab. 2021;36(4):895-903. Published online August 9, 2021
-
DOI: https://doi.org/10.3803/EnM.2021.1026
-
-
6,115
View
-
230
Download
-
4
Web of Science
-
4
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader  ePub ePub
- Background
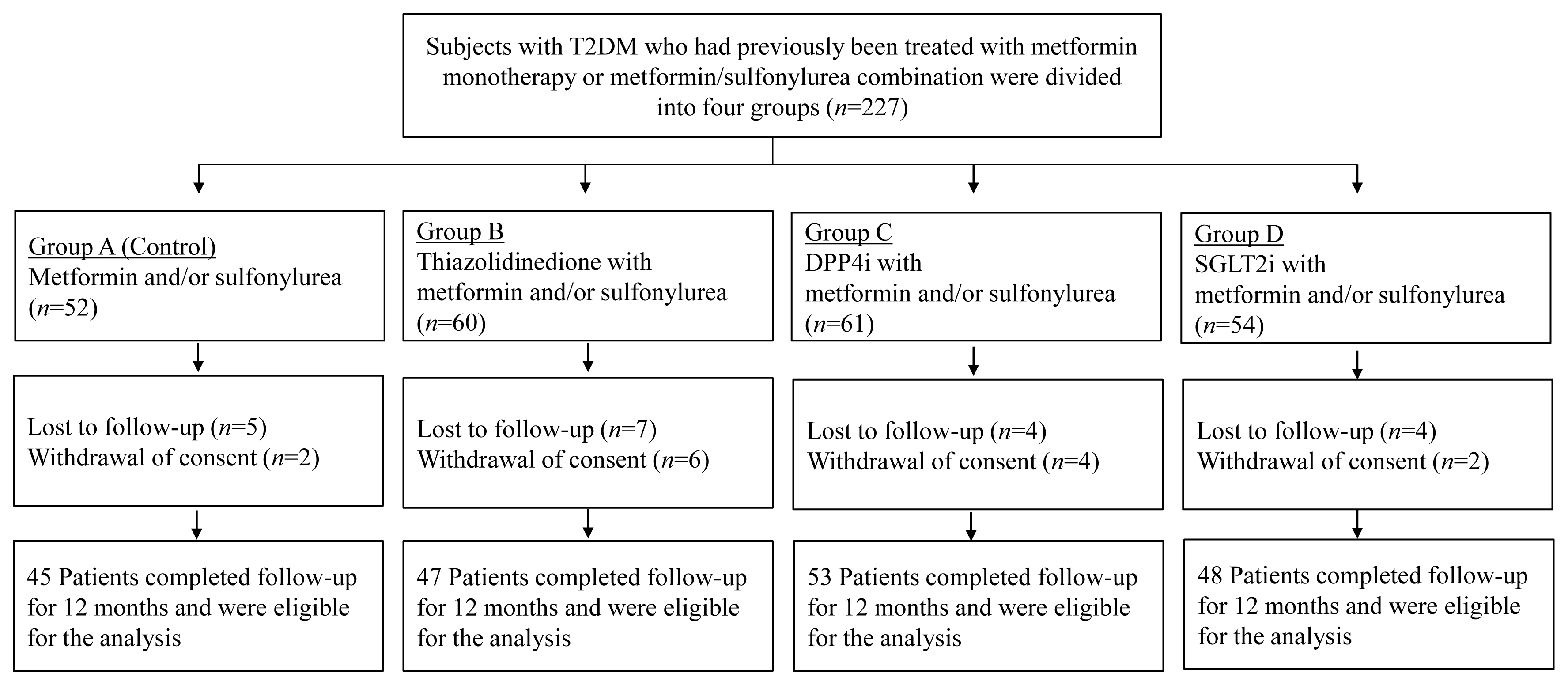
Prospective comparative studies on the effects of various antidiabetic agents on bone metabolism are limited. This study aimed to assess changes in bone mass and biochemical bone markers in postmenopausal patients with type 2 diabetes mellitus (T2DM).
Methods
This prospective, multicenter, open-label, comparative trial included 264 patients with T2DM. Patients who had received a metformin, or sulfonylurea/metformin combination (Group 1); a thiazolidinedione combination (Group 2); a dipeptidyl peptidase-4 inhibitor (gemigliptin) combination (Group 3); or an sodium-glucose cotransporter 2 inhibitor (empagliflozin) combination (Group 4) were prospectively treated for 12 months; bone mineral density (BMD) and bone turnover marker (BTM) changes were evaluated.
Results
The femoral neck BMD percentage changes were −0.79%±2.86% (Group 1), −2.50%±3.08% (Group 2), −1.05%±2.74% (Group 3), and −1.24%±2.91% (Group 4) (P<0.05). The total hip BMD percentage changes were −0.57%±1.79% (Group 1), −1.74%±1.48% (Group 2), −0.75%±1.87% (Group 3), and −1.27%±1.72% (Group 4) (P<0.05). Mean serum BTM (C-terminal type 1 collagen telopeptide and procollagen type 1 amino-terminal propeptide) levels measured during the study period did not change over time or differ between groups.
Conclusion
Significant bone loss in the femoral neck and total hip was associated with thiazolidinedione combination regimens. However, bone loss was not significantly associated with combination regimens including gemigliptin or empagliflozin. Caution should be exercised during treatment with antidiabetic medications that adversely affect the bone in patients with diabetes at a high risk of bone loss.
-
Citations
Citations to this article as recorded by  - Meta-Analysis on the Association Between DPP-4 Inhibitors and Bone Mineral Density and Osteoporosis
Lili Huang, Wei Zhong, Xinghuan Liang, Huijuan Wang, Shi-en Fu, Zuojie Luo
Journal of Clinical Densitometry.2024; 27(1): 101455. CrossRef - A multicentre, double‐blind, placebo‐controlled, randomized, parallel comparison, phase 3 trial to evaluate the efficacy and safety of pioglitazone add‐on therapy in type 2 diabetic patients treated with metformin and dapagliflozin
Soo Lim, Seung‐Hwan Lee, Kyung‐Wan Min, Chang Beom Lee, Sang Yong Kim, Hye Jin Yoo, Nan Hee Kim, Jae Hyeon Kim, Seungjoon Oh, Jong Chul Won, Hyuk Sang Kwon, Mi Kyung Kim, Jung Hwan Park, In‐Kyung Jeong, Sungrae Kim
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - Association of Bone Turnover Markers with Type 2 Diabetes Mellitus and Microvascular Complications: A Matched Case-Control Study
Yilin Hou, Xiaoyu Hou, Qian Nie, Qiuyang Xia, Rui Hu, Xiaoyue Yang, Guangyao Song, Luping Ren
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 1177. CrossRef - Complementary effects of dapagliflozin and lobeglitazone on metabolism in a diet-induced obese mouse model
Yun Kyung Lee, Tae Jung Oh, Ji In Lee, Bo Yoon Choi, Hyen Chung Cho, Hak Chul Jang, Sung Hee Choi
European Journal of Pharmacology.2023; 957: 175946. CrossRef
- Thyroid
- Refractory Graves' Disease Successfully Cured by Adjunctive Cholestyramine and Subsequent Total Thyroidectomy
-
Yeoree Yang, Seawon Hwang, Minji Kim, Yejee Lim, Min-Hee Kim, Sohee Lee, Dong-Jun Lim, Moo-Il Kang, Bong-Yun Cha
-
Endocrinol Metab. 2015;30(4):620-625. Published online December 31, 2015
-
DOI: https://doi.org/10.3803/EnM.2015.30.4.620
-
-
4,405
View
-
85
Download
-
15
Web of Science
-
13
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader
The three major forms of treatment for Graves thyrotoxicosis are antithyroid drugs, radioactive iodine therapy and thyroidectomy. Surgery is the definitive treatment for Graves thyrotoxicosis that is generally recommended when other treatments have failed or are contraindicated. Generally, thyrotoxic patients should be euthyroid before surgery to minimize potential complications which usually requires preoperative management with thionamides or inorganic iodine. But several cases of refractory Graves' disease have shown resistance to conventional treatment. Here we report a 40-year-old female patient with Graves' disease who complained of thyrotoxic symptoms for 7 months. Her thyroid function test and thyroid autoantibody profiles were consistent with Graves' disease. One kind of thionamides and β-blocker were started to control her disease. However, she was resistant to nearly all conventional medical therapies, including β-blockers, inorganic iodine, and two thionamides. She experienced hepatotoxicity from the thionamides. What was worse is her past history of serious allergic reaction to corticosteroids, which are often used to help control symptoms. A 2-week regimen of high-dose cholestyramine improved her uncontrolled thyrotoxicosis and subsequent thyroidectomy was successfully performed. In conclusion, cholestyramine could be administered as an effective and safe adjunctive agent for preoperative preparation in patients with severe hyperthyroid Graves's disease that is resistant to conventional therapies. -
Citations
Citations to this article as recorded by  - Enhanced predictive validity of integrative models for refractory hyperthyroidism considering baseline and early therapy characteristics: a prospective cohort study
Xinpan Wang, Tiantian Li, Yue Li, Qiuyi Wang, Yun Cai, Zhixiao Wang, Yun Shi, Tao Yang, Xuqin Zheng
Journal of Translational Medicine.2024;[Epub] CrossRef - Non-thionamide antithyroid drug options in Graves’ hyperthyroidism
Aliya Ruslan, Onyebuchi E Okosieme
Expert Review of Endocrinology & Metabolism.2023; 18(1): 67. CrossRef - Gestione clinica dell’ipertiroidismo refrattario
Daniela Gallo, Federica Martina Bianchi, Francesca Manzella La Barbera, Ilaria Clementi, Adriana Lai, Eliana Piantanida, Maria Laura Tanda
L'Endocrinologo.2023; 24(2): 167. CrossRef - Carbimazole-Resistant Grave’s Thyrotoxicosis is a Diagnostic and Therapeutic Dilemma, Case Report with Literature Review
Fateen Ata, Adeel Ahmad Khan, Shuja Tahir, Zaina Al Amer
International Medical Case Reports Journal.2023; Volume 16: 783. CrossRef - Medical treatment of thyrotoxicosis
Lorenzo SCAPPATICCIO, Giuseppe BELLASTELLA, Maria I. MAIORINO, Luca GIOVANELLA, Katherine ESPOSITO
The Quarterly Journal of Nuclear Medicine and Molecular Imaging.2021;[Epub] CrossRef - THERAPEUTIC EFFECTS OF COMBINATION REGIMENS INCLUDING METHIMAZOLE ON GRAVES HYPERTHYROIDISM: A NETWORK META-ANALYSIS OF RANDOMIZED CONTROLLED TRIALS
Maorong Wang, Yerong Yu
Endocrine Practice.2020; 26(6): 675. CrossRef - Cholestyramine alters bile acid amounts and the expression of cholesterol‐related genes in rabbit intestinal and hepatic tissues
Dong Ni Qiu, Quan Shang, Da Yu Sun, Wei Qun Ding, Zhong Guang Luo, Jian Chen, Wei Ru Jiang, Jian Ping Huang, Xiao Yun Jiang
Journal of Digestive Diseases.2017; 18(2): 107. CrossRef - ENDOCRINOLOGY IN PREGNANCY: Pregnancy and the incidence, diagnosing and therapy of Graves’ disease
Peter Laurberg, Stine Linding Andersen
European Journal of Endocrinology.2016; 175(5): R219. CrossRef - 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis
Douglas S. Ross, Henry B. Burch, David S. Cooper, M. Carol Greenlee, Peter Laurberg, Ana Luiza Maia, Scott A. Rivkees, Mary Samuels, Julie Ann Sosa, Marius N. Stan, Martin A. Walter
Thyroid.2016; 26(10): 1343. CrossRef - Cholestyramine Use for Rapid Reversion to Euthyroid States in Patients with Thyrotoxicosis
Jeonghoon Ha, Kwanhoon Jo, Borami Kang, Min-Hee Kim, Dong-Jun Lim
Endocrinology and Metabolism.2016; 31(3): 476. CrossRef - The role of bile acids in metabolic regulation
Libor Vítek, Martin Haluzík
Journal of Endocrinology.2016; 228(3): R85. CrossRef - Recent Advances in Autoimmune Thyroid Diseases
Won Sang Yoo, Hyun Kyung Chung
Endocrinology and Metabolism.2016; 31(3): 379. CrossRef - A Case of Methimazole-Resistant Severe Graves' Disease: Dramatic Response to Cholestyramine
Seung Byung Chae, Eun Sook Kim, Yun Im Lee, Bo Ram Min
International Journal of Thyroidology.2016; 9(2): 190. CrossRef
|